Aims and Scope
 Current Cancer Reports (CCR) (eISSN: 2661-3166), published by Syncsci Publishing, is an open access, international, refereed journal published continuously. It focuses on publishing articles covering all domains of carcinogenesis, prevention, diagnosis, treatment, drug development, and relevant policies in oncology.
Current Cancer Reports (CCR) (eISSN: 2661-3166), published by Syncsci Publishing, is an open access, international, refereed journal published continuously. It focuses on publishing articles covering all domains of carcinogenesis, prevention, diagnosis, treatment, drug development, and relevant policies in oncology.
The journal aims at promoting research communications, and providing a platform for doctors, researchers, physicians, pharmacists and healthcare professionals to find the most recent advances in all areas of cancer-related fields. Current Cancer Reports accepts original research articles, reviews, minireviews, case reports, image data, novel hypothesis and rapid communication covering all respects of carcinogenesis and cancer therapy.
The columns of the journal include, but not limited to:
• Original articles and new techniques in cancer research and therapy
• Quick reports
• Case reports
• Clinicopathologic discussion
• Discussion of clinical case
• Expert views
• Exchange of experience
• Novel hypothesis
• Correspondence
• Publish the original incoming letter
• Academic contending/Debate
• etc.
 |
eISSN: 2661-3166
Abbreviation: Curr Cancer Rep
Editor-in-Chief: Prof. Yingyu Cui
Publishing Frequency: Continuous publication
Article Processing Charges (APC): Click here for more details
Publishing Model: Open Access |



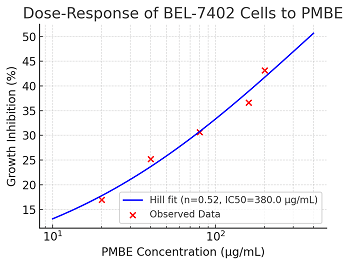
 Ying-Yu Cui, Xi-Han Fang, Xiao-Qing Fu
Ying-Yu Cui, Xi-Han Fang, Xiao-Qing Fu





