Nanotherapeutics to cure inflammation-induced cancer
Main Article Content
Abstract
Aims: Nanotherapeutics are being explored as a potential solution to treat inflammation-induced cancer. Nanotherapeutics enhance innate immune cells' immunity, enabling them to fight tumors effectively. These cells secrete specific chemicals like cytokines, allowing them to replicate quickly and respond to future threats, making them suitable for immunotherapy.
Methods: Nanotechnology can significantly improve human health by enhancing infection detection, prevention, and treatment. Nanomedicines, composed of restorative and imaging compounds in submicrometer-sized materials, aim to deliver effective treatments and limit inflammation in healthy body areas. Combining nanotechnology and clinical sciences, nanoparticles are suitable for gene therapy and have been developed for treating various diseases, including cancer, cardiovascular, diabetes, pulmonary, and inflammatory diseases.
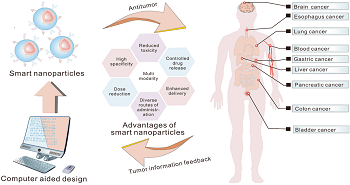
Results: Neutrophils and their offspring, including films and extracellular vehicles, are crucial drug transporters for enhanced growth therapy. Tumor microenvironment inputs can modify tumor-associated neutrophils (TANs), which are essential for tumor growth and healing. Human tumor intratumor heterogeneity is crucial for tumor growth and healing. Nanomedicines have shown potential in targeted delivery, toxicity reduction, and therapeutic effectiveness enhancement. However, clinical relevance and efficacy remain inadequate due to a lack of understanding of the interaction between nanomaterials, nanomedicine, and biology. The diverse biological milieu impacts the dynamic bioidentity of nanoformulations, and their interactions can modify therapeutic function or cellular absorption.
Conclusion: Nanotechnology holds great promise for improving human health by detecting, preventing, and treating infections. Nanomedicines, a fusion of clinical sciences and nanotechnology, use submicrometer-sized transporter materials for therapy delivery and reducing contamination. Nanoparticles' small size and high surface-to-volume ratio can benefit gene therapy. Research has led to a wide range of nanomedicine products globally.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Sun L, Liu H, Ye Y, et al. Smart nanoparticles for cancer therapy. Signal Transduction and Targeted Therapy. 2023, 8(1). https://doi.org/10.1038/s41392-023-01642-x
- Dammes N, Goldsmith M, Ramishetti S, et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nature Nanotechnology. 2021, 16(9): 1030-1038. https://doi.org/10.1038/s41565-021-00928-x
- Caffo M, Curcio A, Rajiv K, et al. Potential Role of Carbon Nanomaterials in the Treatment of Malignant Brain Gliomas. Cancers. 2023, 15(9): 2575. https://doi.org/10.3390/cancers15092575
- Kumar R. SARS-CoV-2, Inflammation, Allergy of the Lungs and Nanotherapeutics. International Journal of Clinical Case Reports and Reviews. 2022, 11(1): 01-02. https://doi.org/10.31579/2690-4861/208
- Kumar R, Chhikara BS, Er Zeybekler S, et al. Nanotoxicity of multifunctional stoichiometric cobalt oxide nanoparticles (SCoONPs) with repercussions toward apoptosis, necrosis, and cancer necrosis factor (TNF-$alpha$) at nano-biointerfaces. Toxicology Research. 2023, 12(5): 716-740. https://doi.org/10.1093/toxres/tfad086
- Hu G, Guo M, Xu J, et al. Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents Against Cancer and Inflammation. Frontiers in Immunology. 2019, 10. https://doi.org/10.3389/fimmu.2019.01998
- Zhang Q, Zhang F, Li S, et al. A Multifunctional Nanotherapy for Targeted Treatment of Colon Cancer by Simultaneously Regulating Tumor Microenvironment. Theranostics. 2019, 9(13): 3732-3753. https://doi.org/10.7150/thno.34377
- Molinaro R, Corbo C, Livingston M, et al. Inflammation and Cancer: In Medio Stat Nano. Current Medicinal Chemistry. 2018, 25(34): 4208-4223. https://doi.org/10.2174/0929867324666170920160030
- Motiei M, Dreifuss T, Betzer O, et al. Differentiating Between Cancer and Inflammation: A Metabolic-Based Method for Functional Computed Tomography Imaging. ACS Nano. 2016, 10(3): 3469-3477. https://doi.org/10.1021/acsnano.5b07576
- Xu M, Hu K, Liu Y, et al. Systemic metastasis-targeted nanotherapeutic reinforces tumor surgical resection and chemotherapy. Nature Communications. 2021, 12(1). https://doi.org/10.1038/s41467-021-23466-5
- Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nature Communications. 2020, 11(1). https://doi.org/10.1038/s41467-020-16439-7
- Xu X, An H, Zhang D, et al. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Science Advances. 2019, 5(1). https://doi.org/10.1126/sciadv.aat2953
- Zhao Q, Jiang D, Sun X, et al. Biomimetic nanotherapy: core–shell structured nanocomplexes based on the neutrophil membrane for targeted therapy of lymphoma. Journal of Nanobiotechnology. 2021, 19(1). https://doi.org/10.1186/s12951-021-00922-4
- Wang H, Liu Y, He R, et al. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomaterials Science. 2020, 8(2): 552-568. https://doi.org/10.1039/c9bm01392j
- Patel P, Meghani N, Kansara K, et al. Nanotherapeutics for the Treatment of Cancer and Arthritis. Current Drug Metabolism. 2019, 20(6): 430-445. https://doi.org/10.2174/1389200220666181127102720
- Zhou H fang, Yan H, Hu Y, et al. Fumagillin Prodrug Nanotherapy Suppresses Macrophage Inflammatory Response via Endothelial Nitric Oxide. ACS Nano. 2014, 8(7): 7305-7317. https://doi.org/10.1021/nn502372n
- Balkwill FR, Mantovani A. Cancer-related inflammation: Common themes and therapeutic opportunities. Seminars in Cancer Biology. 2012, 22(1): 33-40. https://doi.org/10.1016/j.semcancer.2011.12.005
- Pradhan R, Chatterjee S, Hembram KC, et al. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. The Journal of Nutritional Biochemistry. 2021, 92: 108624. https://doi.org/10.1016/j.jnutbio.2021.108624
- Visaria R, Bischof JC, Loren M, et al. Nanotherapeutics for enhancing thermal therapy of cancer. International Journal of Hyperthermia. 2007, 23(6): 501-511. https://doi.org/10.1080/02656730701611241
- Liu YM, Chen YH, Jin YC, et al. Iron based nanotherapeutics for ferroptosis-induced cancer therapy. European Review for Medical & Pharmacological Sciences. 2020, 24(21). https://doi.org/10.26355/eurrev_202011_23623
- Tran S, DeGiovanni P, Piel B, et al. Cancer nanomedicine: a review of recent success in drug delivery. Clinical and Translational Medicine. 2017, 6(1). https://doi.org/10.1186/s40169-017-0175-0
- Li Z, Tan S, Li S, et al. Cancer drug delivery in the nano era: An overview and perspectives. Oncology Reports. 2017, 38(2): 611-624. https://doi.org/10.3892/or.2017.5718
- Amere Subbarao S. Cancer vs. SARS-CoV-2 induced inflammation, overlapping functions, and pharmacological targeting. Inflammopharmacology. 2021, 29(2): 343-366. https://doi.org/10.1007/s10787-021-00796-w
- Mahalunkar S, Kundu GC, Gosavi SW. Folated curcumin-gold nanoformulations: A nanotherapeutic strategy for breast cancer therapy. Journal of Vacuum Science & Technology B. 2020, 38(5): 050802. https://doi.org/10.1116/6.0000148
- Kumar R. Nanotheranostics and Coronavirus 2 (SARS-CoV-2). Biomedical Journal of Scientific & Technical Research. 2021, 37(4). https://doi.org/10.26717/bjstr.2021.37.006050
- Khan MA, Khan MJ. Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artificial Cells, Nanomedicine, and Biotechnology. 2018, 46(sup1): 1149-1158. https://doi.org/10.1080/21691401.2018.1446968
- Sulaiman GM, Waheeb HM, Jabir MS, et al. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Scientific Reports. 2020, 10(1). https://doi.org/10.1038/s41598-020-66419-6
- Zhou J, Li M, Lim WQ, et al. A Transferrin-Conjugated Hollow Nanoplatform for Redox-Controlled and Targeted Chemotherapy of Tumor with Reduced Inflammatory Reactions. Theranostics. 2018, 8(2): 518-532. https://doi.org/10.7150/thno.21194
- Siegler EL, Kim YJ, Wang P. Nanomedicine targeting the tumor microenvironment: Therapeutic strategies to inhibit angiogenesis, remodel matrix, and modulate immune responses. Journal of Cellular Immunotherapy. 2016, 2(2): 69-78. https://doi.org/10.1016/j.jocit.2016.08.002
- Düzgüneş N. Preface. Nanomedicine - Cancer, Diabetes, and Cardiovascular, Central Nervous System, Pulmonary and Inflammatory Diseases. Published online 2012: xix-xxi. https://doi.org/10.1016/b978-0-12-391860-4.00027-6
- Chen Q, Liang C, Sun X, et al. H 2 O 2 -responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proceedings of the National Academy of Sciences. 2017, 114(21): 5343-5348. https://doi.org/10.1073/pnas.1701976114
- Feng L, Dong Z, Tao D, et al. The acidic tumor microenvironment: a target for smart cancer nano-theranostics. National Science Review. 2017, 5(2): 269-286. https://doi.org/10.1093/nsr/nwx062
- Al-Hazmi NE, Naguib DM. Nano-peroxidase a Promising Anti-inflammatory and Antibacterial Agent Against Bacteria and Inflammation Related to Colorectal Cancer. Journal of Gastrointestinal Cancer. 2021, 53(2): 415-419. https://doi.org/10.1007/s12029-021-00626-w
- Chao BH, Bischof JC. Pre-Existing Inflammation Induced by TNF-Alpha Augments Cryosurgery on Human Prostate Cancer. Heat Transfer, Volume 4. Published online 2003. https://doi.org/10.1115/imece2003-41955
- Chowdhury P, Ghosh U, Samanta K, et al. Bioactive nanotherapeutic trends to combat triple negative breast cancer. Bioactive Materials. 2021, 6(10): 3269-3287. https://doi.org/10.1016/j.bioactmat.2021.02.037
- Kiraly O, Gong G, Olipitz W, et al. Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations In Vivo. Risques R, ed. PLOS Genetics. 2015, 11(2): e1004901. https://doi.org/10.1371/journal.pgen.1004901
- Chowdhury EH. Nanotherapeutics. Published online April 21, 2016. https://doi.org/10.1201/b19573
- Talekar M, Tran TH, Amiji M. Translational Nano-Medicines: Targeted Therapeutic Delivery for Cancer and Inflammatory Diseases. The AAPS Journal. 2015, 17(4): 813-827. https://doi.org/10.1208/s12248-015-9772-2
- Bhaskar S, Singh MS. Nanocarrier-based immunotherapy in cancer management and research. ImmunoTargets and Therapy. Published online June 2014: 121. https://doi.org/10.2147/itt.s62471
- Ray P, Haideri N, Haque I, et al. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. Journal of Immunological Sciences. 2021, 5(1): 19-33. https://doi.org/10.29245/2578-3009/2021/1.1206
- Wu Z, Huang D, Wang J, et al. Engineering Heterogeneous Tumor Models for Biomedical Applications. Advanced Science. 2023, 11(1). https://doi.org/10.1002/advs.202304160
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer. 2016, 17(1): 20-37. https://doi.org/10.1038/nrc.2016.108
- Tang L, Wang Z, Mu Q, et al. Targeting Neutrophils for Enhanced Cancer Theranostics. Advanced Materials. 2020, 32(33). https://doi.org/10.1002/adma.202002739
- Torres Andón F, Alonso MJ. Nanomedicine and cancer immunotherapy – targeting immunosuppressive cells. Journal of Drug Targeting. 2015, 23(7-8): 656-671. https://doi.org/10.3109/1061186x.2015.1073295
- Nanomedicine for Inflammatory Diseases. CRC Press, 2017. https://doi.org/10.1201/978131515235
- Song S, Xia H, Guo M, et al. Role of macrophage in nanomedicine-based disease treatment. Drug Delivery. 2021, 28(1): 752-766. https://doi.org/10.1080/10717544.2021.1909175
- Marcos-Contreras OA, Greineder CF, Kiseleva RY, et al. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood–brain barrier. Proceedings of the National Academy of Sciences. 2020, 117(7): 3405-3414. https://doi.org/10.1073/pnas.1912012117
- Zhou Y, Zhang N, Dai Z. Advances in enhancing cancer immunotherapy by nanotechnology. Chinese Science Bulletin. 2018, 63(5-6): 535-546. https://doi.org/10.1360/n972017-01059
- Du S, Guan Y, Xie A, et al. Extracellular vesicles: a rising star for therapeutics and drug delivery. Journal of Nanobiotechnology. 2023, 21(1). https://doi.org/10.1186/s12951-023-01973-5
- Liu Q, Li D, Pan X, et al. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. Journal of Nanobiotechnology. 2023, 21(1). https://doi.org/10.1186/s12951-023-02081-0
- Que H, Fu Q, Lan T, et al. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2022, 1877(5): 188762. https://doi.org/10.1016/j.bbcan.2022.188762
- Ng MSF, Kwok I, Tan L, et al. Deterministic reprogramming of neutrophils within tumors. Science. 2024, 383(6679). https://doi.org/10.1126/science.adf6493
- Gungabeesoon J, Gort-Freitas NA, Kiss M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 2023, 186(7): 1448-1464.e20. https://doi.org/10.1016/j.cell.2023.02.032
- Mbeunkui F, Johann DJ. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemotherapy and Pharmacology. 2008, 63(4): 571-582. https://doi.org/10.1007/s00280-008-0881-9
- Zhang J, Gu J, Wang X, et al. Engineering and Targeting Neutrophils for Cancer Therapy. Advanced Materials. 2024, 36(19). https://doi.org/10.1002/adma.202310318
- Sanegre S, Lucantoni F, Burgos-Panadero R, et al. Integrating the Tumor Microenvironment into Cancer Therapy. Cancers. 2020, 12(6): 1677. https://doi.org/10.3390/cancers12061677
- McCune J. Immunotherapy to Treat Cancer. Clinical Pharmacology & Therapeutics. 2016, 100(3): 198-203. https://doi.org/10.1002/cpt.404
- Xie N, Shen G, Gao W, et al. Neoantigens: promising targets for cancer therapy. Signal Transduction and Targeted Therapy. 2023, 8(1). https://doi.org/10.1038/s41392-022-01270-x
- Schumacher T, Bunse L, Pusch S, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014, 512(7514): 324-327. https://doi.org/10.1038/nature13387
- Darragh LB, Karam SD. Amateur antigen‐presenting cells in the tumor microenvironment. Molecular Carcinogenesis. 2021, 61(2): 153-164. https://doi.org/10.1002/mc.23354
- Hinohara K, Polyak K. Intratumoral Heterogeneity: More Than Just Mutations. Trends in Cell Biology. 2019, 29(7): 569-579. https://doi.org/10.1016/j.tcb.2019.03.003
- Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. British Journal of Cancer. 2013, 108(3): 479-485. https://doi.org/10.1038/bjc.2012.581
- Simmons D. Epigenetic Influences and Disease. National Education. 2008, 1.
- Song H, Shen R, Mahasin H, et al. DNA replication: Mechanisms and therapeutic interventions for diseases. MedComm. 2023, 4(1). https://doi.org/10.1002/mco2.210
- Jančík S, Drábek J, Radzioch D, et al. Clinical Relevance of KRAS in Human Cancers. Journal of Biomedicine and Biotechnology. 2010, 2010: 1-13. https://doi.org/10.1155/2010/150960
- Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. Journal of Hematology & Oncology. 2019, 12(1). https://doi.org/10.1186/s13045-019-0818-2
- Lin Y, Chen Y, Luo Z, et al. Recent advances in biomaterial designs for assisting CAR-T cell therapy towards potential solid tumor treatment. Nanoscale. 2024, 16(7): 3226-3242. https://doi.org/10.1039/d3nr05768b
- Galon J, Bruni D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity. 2020, 52(1): 55-81. https://doi.org/10.1016/j.immuni.2019.12.018
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. Journal of Molecular Medicine. 2020, 98(2): 161-177. https://doi.org/10.1007/s00109-020-01874-2
- Lynch I, Feitshans IL, Kendall M. ‘Bio-nano interactions: new tools, insights and impacts’: summary of the Royal Society discussion meeting. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015, 370(1661): 20140162. https://doi.org/10.1098/rstb.2014.0162
- Wang Y, Cai R, Chen C. The Nano–Bio Interactions of Nanomedicines: Understanding the Biochemical Driving Forces and Redox Reactions. Accounts of Chemical Research. 2019, 52(6): 1507-1518. https://doi.org/10.1021/acs.accounts.9b00126
- Tian X, Chong Y, Ge C. Understanding the Nano–Bio Interactions and the Corresponding Biological Responses. Frontiers in Chemistry. 2020, 8. https://doi.org/10.3389/fchem.2020.00446
- Liu J, Guo M, Chen C. Nano-bio interactions: A major principle in the dynamic biological processes of nano-assemblies. Advanced Drug Delivery Reviews. 2022, 186: 114318. https://doi.org/10.1016/j.addr.2022.114318
- Tuguntaev RG, Hussain A, Fu C, et al. Bioimaging guided pharmaceutical evaluations of nanomedicines for clinical translations. Journal of Nanobiotechnology. 2022, 20(1). https://doi.org/10.1186/s12951-022-01451-4
- Manheim DC, Detwiler RL. Accurate and reliable estimation of kinetic parameters for environmental engineering applications: A global, multi objective, Bayesian optimization approach. MethodsX. 2019, 6: 1398-1414. https://doi.org/10.1016/j.mex.2019.05.035
- Joseph T, Kar Mahapatra D, Esmaeili A, et al. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials. 2023, 13(3): 574. https://doi.org/10.3390/nano13030574
- Doroudian M, MacLoughlin R, Poynton F, et al. Nanotechnology based therapeutics for lung disease. Thorax. 2019, 74(10): 965-976. https://doi.org/10.1136/thoraxjnl-2019-213037
- Zafar F, Jahan N, Khalil-Ur-Rahman, et al. Nanosuspension enhances dissolution rate and oral bioavailability of Terminalia arjuna bark extract in vivo and in vitro. Asian Pacific Journal of Tropical Biomedicine. 2020, 10(4): 164. https://doi.org/10.4103/2221-1691.280293
- Gulumian M, Andraos C, Afantitis A, et al. Importance of Surface Topography in Both Biological Activity and Catalysis of Nanomaterials: Can Catalysis by Design Guide Safe by Design? International Journal of Molecular Sciences. 2021, 22(15): 8347. https://doi.org/10.3390/ijms22158347
- Sun D, Zhou S, Gao W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano. 2020, 14(10): 12281-12290. https://doi.org/10.1021/acsnano.9b09713
- Pateiro M, Gómez B, Munekata PES, et al. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules. 2021, 26(6): 1547. https://doi.org/10.3390/molecules26061547
- Desai N. Challenges in Development of Nanoparticle-Based Therapeutics. The AAPS Journal. 2012, 14(2): 282-295. https://doi.org/10.1208/s12248-012-9339-4
- Olawore O, Ogunmola M, Desai S. Engineered Nanomaterial Coatings for Food Packaging: Design, Manufacturing, Regulatory, and Sustainability Implications. Micromachines. 2024, 15(2): 245. https://doi.org/10.3390/mi15020245
- Kumari A, Singla R, Guliani A, et al. Nanoencapsulation for drug delivery. EXCLI journal. 2014, 13: 265.
- Yang C, Merlin D. Challenges to Safe Nanomedicine Treatment. Nanomaterials. 2023, 13(7): 1171. https://doi.org/10.3390/nano13071171
- Li Y, Meng Q, Yang M, et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharmaceutica Sinica B. 2019, 9(6): 1113-1144. https://doi.org/10.1016/j.apsb.2019.10.001