Clarifying Aromaticity and Delocalization with the Principle of π-Electron Pair Interaction (PEPI)
Main Article Content
Abstract
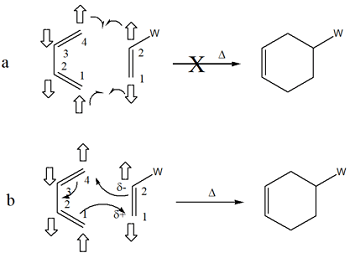
Valence bond (VB) theory and Molecular Orbital (MO) theory are foundational approaches to understanding chemical bonding. While MO theory describes delocalized orbitals across the molecule and offers quantitative rigor, VB theory aligns closely with classical chemical concepts, using localized bonds and hybridization for intuitive understanding. However, VB theory’s treatment of delocalized systems, such as aromatic compounds, relies on resonance structures, which are less efficient and may cause misconceptions compared to the methodology of MO theory. To address this, the Principle of π-Electron Pair Interaction (PEPI) is introduced as a heuristic framework to extend the qualitative power of VB theory. A visual guide is provided by PEPI to aid in understanding when π-electrons may resist delocalization due to pairing constraints. The model is intended to complement MO theory and is presented not as a physical principle, but as an interpretive aid that offers clarity in systems such as butadiene, benzene, and selected pericyclic reactions. It is demonstrated how PEPI can illuminate concepts such as aromaticity, antiaromaticity, misinterpretations of resonance, and stereoelectronic trends in a conceptually accessible manner. PEPI proposes that electron spin should be taken into account when evaluating resonance structures, particularly in the context of aromaticity. By reframing PEPI as a pedagogical tool, alignment is achieved with quantum mechanical models while the intuitive appeal of VB theory is preserved.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Murrell JN, Kettle SFA, Tedder JM. The Chemical Bond (2nd ed.). 1985. John Wiley & Sons. ISBN 0-471-90759-6.
- Xu LT, Cooper DL, Dunning TH. Resolving a puzzling anomaly in the spin-coupled generalized valence bond description of benzene. Journal of Computational Chemistry. 2020, 41(15): 1421-1426. https://doi.org/10.1002/jcc.26185
- Xu LT, Dunning TH. Orbital Hybridization in Modern Valence Bond Wave Functions: Methane, Ethylene, and Acetylene. The Journal of Physical Chemistry A. 2019, 124(1): 204-214. https://doi.org/10.1021/acs.jpca.9b11054
- Radenković S, Danovich D, Shaik S, et al. The nature of bonding in metal-metal singly bonded coinage metal dimers: Cu2, Ag2 and Au2. Computational and Theoretical Chemistry. 2017, 1116: 195-201.
- Shaik S, Danovich D, Galbraith JM, et al. Charge-Shift Bonding: A New and Unique Form of Bonding. Angewandte Chemie International Edition. 2019, 59(3): 984-1001. https://doi.org/10.1002/anie.201910085
- Bury CR. Langmuir’s Theory of the Arrangement of Electrons in Atoms and Molecules. Journal of the American Chemical Society. 1921, 43(7): 1602-1609. https://doi.org/10.1021/ja01440a023
- Cooper DL, Gerratt J, Raimondi M. The electronic structure of the benzene molecule. Nature. 1986, 323(6090): 699-701. https://doi.org/10.1038/323699a0
- Vemulapalli GK. Theories of the chemical bond and its true nature. Foundations of Chemistry. 2008, 10(3): 167-176. https://doi.org/10.1007/s10698-008-9049-2
- Shurki A, Hiberty PC, Dijkstra F, et al. Aromaticity and antiaromaticity: what role do ionic configurations play in delocalization and induction of magnetic properties? Journal of Physical Organic Chemistry. 2003, 16(10): 731-745. https://doi.org/10.1002/poc.658
- Karadakov PB, Cooper DL, Gerratt J. Modern Valence-Bond Description of Chemical Reaction Mechanisms: Diels-Alder Reaction. Journal of the American Chemical Society. 1998, 120(16): 3975-3981. https://doi.org/10.1021/ja9741741
- Shaik S, Danovich D, Hiberty PC. Valence Bond Theory-Its Birth, Struggles with Molecular Orbital Theory, Its Present State and Future Prospects. Molecules. 2021, 26(6): 1624. https://doi.org/10.3390/molecules26061624
- Woodward RB, Hoffmann R. The Conservation of Orbital Symmetry. Angewandte Chemie International Edition in English. 1969, 8(11): 781-853. https://doi.org/10.1002/anie.196907811
- Fukui K, Yonezawa T, Shingu H. A molecular orbital theory of reactivity in aromatic hydrocarbons. The Journal of Chemical Physics. 1952, 20(4): 722-725.
- Dunning TH, Xu LT, Cooper DL, et al. Spin-Coupled Generalized Valence Bond Theory: New Perspectives on the Electronic Structure of Molecules and Chemical Bonds. The Journal of Physical Chemistry A. 2021, 125(10): 2021-2050. https://doi.org/10.1021/acs.jpca.0c10472
- Eisberg R, Resnick R. Quantum Physics of Atoms, Molecules, Solids, Nuclei, and Particles (2nd ed.). 1985. Wiley. pp. 272–273.
- Gorbar EV, Miranskij VA, Shovkovy IA, et al. Electronic Properties of Dirac and Weyl Semimetals. 2021, World Scientific Publishing.
- Krane KS. Introductory Nuclear Physics. Wiley. 1987. ISBN 978-0-471-80553-3.
- Houk KN, Lin YTsong, Brown FK. Evidence for the concerted mechanism of the Diels-Alder reaction of butadiene with ethylene. Journal of the American Chemical Society. 1986, 108(3): 554-556. https://doi.org/10.1021/ja00263a059
- Goldstein E, Beno B, Houk KN. Density Functional Theory Prediction of the Relative Energies and Isotope Effects for the Concerted and Stepwise Mechanisms of the Diels-Alder Reaction of Butadiene and Ethylene. Journal of the American Chemical Society. 1996, 118(25): 6036-6043. https://doi.org/10.1021/ja9601494
- Dewar MJS, Olivella Santiago, Stewart JJP. Mechanism of the Diels-Alder reaction: reactions of butadiene with ethylene and cyanoethylenes. Journal of the American Chemical Society. 1986, 108(19): 5771-5779. https://doi.org/10.1021/ja00279a018