Benefits and problems of chrome tanning in leather processing: Approach a greener technology in leather industry
Main Article Content
Abstract
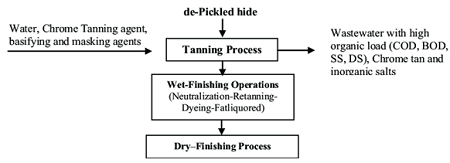
Tanning is the process of converting the raw skin and hides from different animals into a sustainable and manageable material called leather. Leather making is a very long process and consists of many different chemical and mechanical process steps. The most important step of the whole leather making process is the tanning step, which is performed commonly either by vegetable or mineral tanning. More than 85-90% of the leather making is performed by chrome tanning, which is the most common type of mineral tanning currently applied.
Keywords
chrome tanning, greener technology, leather processing
Article Details
How to Cite
Ahmed, M. D., & Maraz, K. M. (2021). Benefits and problems of chrome tanning in leather processing: Approach a greener technology in leather industry. Materials Engineering Research, 3(1), 156-164. https://doi.org/10.25082/MER.2021.01.004

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Parivallal B and Ramanujam R. Studies on degradation of suntan used in leather tanning process using ozone. International Journal of Environmental Science and Development, 2010, 53: 264-267. https://doi.org/10.7763/IJESD.2010.V1.51
- Krishnamoorthy G, Sadulla S, Sehgal P, et al. Greener approach to leather tanning process: d-Lysine aldehyde as novel tanning agent for chrome-free tanning. Journal of Cleaner Production, 2013, 42: 277-286. https://doi.org/10.1016/j.jclepro.2012.11.004
- Theis E and Goetz A. Chrome Tanning I. The role played by sodium chloride in chrome liquors upon chrome tanning. Industrial & Engineering Chemistry, 1932, 24(3): 304-307. https://doi.org/10.1021/ie50267a009
- Nashy E and Eid K. Reduction of chrome tanning effluent impact and enhancement of leather properties based on high exhaustion of chrome tan. Egyptian Journal of Chemistry, 2018, 5: 12-18. https://doi.org/10.21608/ejchem.2018.4393.1387
- Puccini M and Castiello D. Use of glucose to improve the environmental aspects of chrome tanning trocess. Advanced Materials Research, 2014, 933: 144-150. https://doi.org/10.4028/www.scientific.net/AMR.933.144
- Gondim R, Marinho R and Conceic¸ ˜ao R. Tanning handmade leather tilapia (oreochomis sp.) from three natural tanning. Revista Brasileira de Higiene e Sanidade Animal, 2015, 9(2): 172-184. https://doi.org/10.5935/1981-2965.20150016
- Sakmat J, Lopattananon N and Kaesaman A. Effect of fiber surface modification on properties of artificial leather from leather fiber filled natural rubber composites. Key Engineering Materials, 2015, 659: 378-382. https://doi.org/10.4028/www.scientific.net/KEM.659.378
- Das S. Animal husbandry practices of livestock keepers in mizoram: a case study. International Journal of Bio-resource and Stress Management, 2018, 9(5): 580-584. https://doi.org/10.23910/IJBSM/2018.9.5.1900
- Maria J. Festive meals in the late middle ages. An essay on dining as a means of communication. Food and History, 2003, 1: 95-102. https://doi.org/10.1484/J.FOOD.2.300505
- Snooks G. A general theory of complex living systems: Exploring the demand side of dynamics. Complexity, 2008, 13(6): 12-20. https://doi.org/10.1002/cplx.20225
- Jhonson R. Sealing material for automotive applications. Sealing Technology, 2018, 5: 13-21. https://doi.org/10.1016/S1350-4789(18)30221-6
- Theis E and Goetz A. Chrome Tanning I. The role played by sodium chloride in chrome liquors upon chrome tanning. Industrial & Engineering Chemistry, 1932, 24(3): 304-307. https://doi.org/10.1021/ie50267a009
- Wilson J and Kern E. Nature of the hide-tannin compound and its bearing upon tannin analysis. Journal of Industrial & Engineering Chemistry, 1920, 12(12): 1149-1151. https://doi.org/10.1021/ie50132a008
- Qin A and Kalemkerian G. Treatment options for relapsed small-cell lung cancer: What progress have we made?. Journal of Oncology Practice, 2018, 14(6): 369-370. https://doi.org/10.1200/JOP.18.00278
- El-Khateeb M, Nashy E, Ghany N, et al. Environmental impact elimination of chrome tanning effluent using electrocoagulation process assisted by chemical oxidation. Desalination and Water Treatment, 2017, 65: 147-152. https://doi.org/10.5004/dwt.2017.20250
- Kasim A, Novia D, Mutiar S, et al. Diminishing chromium use on combined chromium-gambier tanning process upon the characteristics of tanned leather. Media Peternakan, 2014, 37(1): 24-29. https://doi.org/10.5398/medpet.2014.37.1.24
- Suresh V, Kanthimathi M, Thanikaivelan P, et al. An improved product-process for cleaner chrome tanning in leather processing. Journal of Cleaner Production, 2001, 9(6): 483-491. https://doi.org/10.1016/S0959-6526(01)00007-5
- Bowker R and Emley W. Comparative wear of chrome-tanned, vegetable-tanned, and retanned sole leather. Journal of Research of the National Bureau of Standards, 1935, 15(4): 363-368. https://doi.org/10.6028/jres.015.022
- Ebrahiem M, Turki I and Haroun H. The effect of breed on skin/leather quality of sudan desert sheep. Journal of Africa Leather and Leather Producuts Advances, 2014, 1(1): 45-53. https://doi.org/10.15677/jallpa.2014.v1i1.5
- Vasovi´c D and Stojakovi´c D. Preparation of chromium(III) phthalate and chromium(III) pyromellitate via chromium(VI) oxide. Journal of Coordination Chemistry, 1992, 25(3): 221-227. https://doi.org/10.1080/00958979209409194
- Ellis A. Chromium isotopes and the fate of hexavalent chromium in the environment. Science, 2002, 295(5562): 2060-2062. https://doi.org/10.1126/science.1068368
- Abdel-Shafy H, Hegemann W and Genschow E. Fate of heavy metals in the leather tanning industrial wastewater using an anaerobic process. Environmental Management and Health, 1995, 6(2): 28-33. https://doi.org/10.1108/09566169510085135
- Kasim A, Novia D, Mutiar S, et al. Diminishing chromium use on combined chromium-gambier tanning process upon the characteristics of tanned leather. Media Peternakan, 2014, 37(1): 24-29. https://doi.org/10.5398/medpet.2014.37.1.24
- Tremper K. Hemoglobin-based oxygen carrying solutions: Will they replace red blood cells?. Anesthesia & Analgesia, 2005, 100(4): 910-911. https://doi.org/10.1213/01.ANE.0000149596.56181.75
- Srivastava S and Srivastava S. New biologically active constituents from Terminalia Chebula stem bark. ChemInform, 2005, 36(17): 2731-2733. https://doi.org/10.1002/chin.200517197
- Udkhiyati M and Silvianti F. The utilization of chitosan as natural antibacterial for vegetable tanned leather. Materials Science Forum, 2019, 948: 212-216. https://doi.org/10.4028/www.scientific.net/MSF.948.212
- Simi´c M. Complex impedance measurement system for the frequency range from 5 kHz to 100 kHz. Key Engineering Materials, 2015, 644: 133-136. https://doi.org/10.4028/www.scientific.net/KEM.644.133
- Theis E and Goetz A. Chrome tanning I. The role played by sodium chloride in chrome liquors upon chrome tanning. Industrial & Engineering Chemistry, 1932, 24(3): 304-307. https://doi.org/10.1021/ie50267a009
- Teng B, Jian X and Chen W. Effect of thallic acid content on tannin-titanium (III) combination tanning. Leather and Footwear Journal, 2013, 13(1): 3-12. https://doi.org/10.24264/lfj.13.1.1
- Liu S, Tong X and Song B. A compact and efficient technology to treat tailings water characterized by high alkalinity and Ca(II) and Pb(II) concentration. Advanced Materials Research, 2012, 518-523: 2278-2282. https://doi.org/10.4028/www.scientific.net/AMR.518-523.2278
- Madera C, Pe˜na M and Mara D. Microbiological quality of a waste stabilization pond effluent used for restricted irrigation in Valle Del Cauca, Colombia. Water Science and Technology, 2002, 45(1): 139-143. https://doi.org/10.2166/wst.2002.0019
- Fox K. Water treatment and equipment decontamination techniques. Journal of Contemporary Water Research & Education, 2009, 129(1): 18-21. https://doi.org/10.1111/j.1936-704X.2004.mp129001005.x
- Gustavson K. Fixation of constituents of chrome liquors by hide substance from highly concentrated chrome solutions. Industrial & Engineering Chemistry, 1925, 17(8): 823-826. https://doi.org/10.1021/ie50188a018