Frumkin-melik-gaykazan model in the potentiodynamic and galvanodynamic regimes of functioning
Main Article Content
Abstract
Aim The main purpose of this article is to study the behavior of a metallic electrode in the electrolyte which contains a surface-active substance with the property of adsorbtion-desorbtion, in the galvanodynamic and potentiodynamic rejimes.
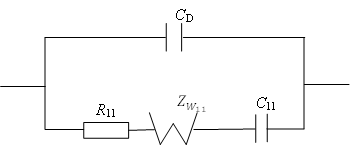
Method The study of the electrochemical behavior of a metallic electrode is carried out by operational impedance method, based on the Ohm’s low on the interaction between the Laplace-transformed expression of current, voltage and complex resistance (impedance) .
Results It is obtained the analytical expression of interface voltage –time dependence in a solution which contains a surface-active indifferent substance with the property of adsorbtion-desorbtion; also it is obtained the analytical expression of current density-time dependence which is passing through electrochemical cell in potentiodynamic regime of functioning of the Frumkin-Melik-Gaykazan model.
Conclusion It is established that the relation between the interface metallic electrode – indifferent electrolyte with property of adsorbtion-desorbtion voltage in the galvanodynamic rejime has the character of second order parabola; the relation between current density which is passing through a cell and time in potentiodynamic rejime of functioning in the Frumkin – Melik – Gaykazan model has exponential character.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Frumkin F.N. Selected Transactions. Electrode Processes. Article in the book: The determination of a adsorbtiom kinetic of the organic substances by method of measuring of capacitance and conduction of electrode-solution interface by alternating current method. M.: Nauka, 1987, p.p. 291-295. (In Russian).
- Frumkin A.N/, Melik – Gaykazan V.I. Doklady of Akademy of Sciences of USSR. 1951, vol.77, №5, pp.855-858. (In Russian).
- Damaskin B.B., Petry O.A. Introduction to the Electrochemical Kinetics. M.: Vyschaya Shkola, 175.- p.143. (In Russian).
- Grafov B.M., Ukshe E.A. The Electrochemical circuites of alternating current. M.: Nauka, 1973, pp.28-32. (In Russian).
- Kontorovich M.I. The Operational calculus and Processes in Electrical Circuites. M.: Soviet Radio, 1975.- 320 p. (In Russian).
- Hanbook on Spesial Functions. Eds.M.Abramovich and I.Stigan. M.: Nauka, 1979. 830 p. (In Russian).
- Dech G. Rukovodstvo k prakticheskomu primeneniy preobrazovaniia Laplasa. Moscow: Nauka Publ., 1965, 287 p. (In Russian).