Vol 6 No 1 (2024)
Research Article
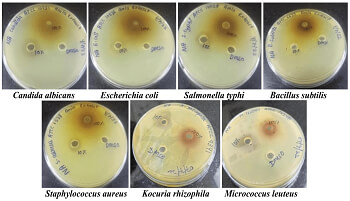
Phyllanthus emblica: A boon or bane - Unlocking the phytopharmaceutical profile
Herbal medicines have been utilised since ancient times to treat various diseases. Medicinal plants have played a significant role in global health, and despite the remarkable progress in modern medicine, plants continue to make a valuable contribution to health. Plants are abundant in tropical regions around the world. Recently, there has been a growing interest in drugs derived from higher plants, especially those used in phytotherapy. It is estimated that about 25% of all modern medicines are directly or indirectly derived from higher plants. In nature, herbs contain a variety of well-arranged medicinal properties. Their uniform medicinal compounds make herbal medicines more effective and of higher quality. Phyllanthus emblica has all the antidiabetic, antimicrobial, DPPH activity and chemical profiling for its potential under the Quality control assessment.
In the present study, the sturdy root of Nigerian Vigna radiata (L.) commonly called mung bean was investigated for the phytochemical content. This was necessitated as a result of limited information observed on the phytochemical content of fully matured Nigerian Vigna radiata root. 60 days old Vigna radiata (L.) plant, a newly introduced crop in Nigeria, was harvested from the farm of National Biotechnology and Research Development Agency, Abagana Centre, Nigeria. The roots were neatly separated from the plant, rinsed well with distilled water, air dried and grounded into flour. The 60 days old Nigerian Vigna radiata root flour sample (NVrR) subjected to preliminary phytochemical assay revealed the presence of 12 bioactive compounds with a remarkable high percentage concentration of 26.780% recorded for flavonoids content. Tannins also recorded appreciable value of 8.927% while values < 5% were noted for the remaining compounds. Further confirmation of the actual bioactive compounds present in 60 days old NVrR through GC-MS studies, generated 30 observable peaks with 28 bioactive compounds identified through spectrum matching with MassHunter\Library\NIST14.L spectral database. The major component, eluted at RT 23.565 (peak area 33.38%) revealed a bioactive compound which has been reported as an active ingredient in the production of detergents and biodiesel. This discovery represents a groundbreaking innovation in the utilization of NVrR for the production of briquettes, offering a cost-effective alternative energy source. Isolation of the identified compounds may prove the NVrR an important raw material for industrial productions.
Leaves of Vigna radiata (L.) are regarded as by-products due to the relatively low emphasis attributed to them when compared to the seeds and sprouts. They are usually left on the farm as waste products or for animals to graze on them, especially here in Nigeria; therefore, the need to investigate its various phytochemical content emerged, which will result in its optimum utilization. In the present study, 60 days old Vigna radiata (L.) leaves were harvested from V. radiata plants cultivated at the National Biotechnology and Research Development Centre Abagana, Anambra State, Nigeria. They were processed and milled into flour. Part of the milled flour was subjected to preliminary quantitative phytochemical screening, which revealed the presence of steroids as the major phytochemical content out of 12 bioactive compounds assessed with a value of 19.298%. The gas chromatography – mass spectrometry (GC-MS) used to determine the actual bioactive compounds present in the methanolic extracts of Nigerian V. radiata leaves (MENVrL) revealed the presence of 53 bioactive compounds with 58 peaks, covering a total peak area of 100% and these compounds were identified through spectrum matching with National Institute Standard and Technology (NIST) database. 2,4-Di-tert-butylphenol was identified as the major compound present in MENVrL with a peak area of 7.66%. Further isolation of these bioactive compounds may prove the leaves a rich source of pharmaceutical, biological, and cosmetologically important raw materials, for the formulation of new effective drugs and other related products.
Review
The clinical and regulatory status of NDSRI: A global imperative
Detecting N-nitrosamine impurities in medicines has been a significant challenge for drug manufacturers and regulators, especially with the recent emergence of nitrosamine drug substance-related impurities (NDSRIs). The formation of NDSRIs is complex and primarily associated with reactions in the drug product. This paper explores the current technical knowledge on forming these impurities, including the risk factors, reaction conditions, and possible mitigation strategies. While significant scientific progress has been made in these areas, substantial gaps in mechanistic knowledge still make accurate predictions of NDSRI formation very difficult. The pharmaceutical industry's continued work on potential mitigation strategies and the generation of additional scientific data to address these knowledge gaps are crucial. Regulatory guidance and policy will continue to evolve in response to further changes in scientific understanding. In this article, we will delve into the detection methods, the mechanism of action, sample preparation techniques, and regulatory limits for nitrosamine impurities. We also discuss various reported nitrosamine impurities, their chemical structures, and their detection using methods like LC-MS/MS, GC-MS-HS, and HPLC. Additionally, we discuss different sample preparation techniques, such as solid-phase extraction, liquid-liquid extraction, and rapid-fire techniques. This review is intended to provide detailed information to analytical personnel working in various quality control laboratories and research organizations.


 Ritu Tiwari, Gaurav Sanjay Mahalpure, Sakshi Mahalpure, Anuanshika Tiwari
Ritu Tiwari, Gaurav Sanjay Mahalpure, Sakshi Mahalpure, Anuanshika Tiwari



